Category 9 Irb
Under the Basel II guidelines banks are allowed to use their own estimated risk parameters for the purpose of calculating regulatory capitalThis is known as the internal ratings-based IRB approach to capital requirements for credit riskOnly banks meeting certain minimum conditions disclosure requirements and approval from their national supervisor are allowed to use this approach in. EXPEDITED CATEGORIES IN NEW COMMON RULE All Pepperdine University human subjects research projects must undergo review and approval by an IRB prior to initiation of research activities.
3 Levels Of Irb Review Committee On The Use Of Human Subjects
Category 9 does not include research conducted under an investigational new drug application.
Category 9 irb. Determining and Documenting Category 9 and the Approval Period. Regulations require IRB review and approval for research involving human subjects if it is funded or regulated by the federal. Continuing review of research not conducted under an investigational new drug application or investigation device exemption where categories two 2 through eight 8 do not apply but the IRB has determined and documented at a convened.
Research activities that 1 present no more than minimal risk to human subjects and 2 involve only procedures listed in one or more of the following categories may be reviewed by the IRB through the expedited review procedure authorized by 45 CFR 46110. The research is not conducted under an investigational new drug application IND or an investigational device exemption IDE. Weight Other technical data Power consumption Path E1-E2-E3-E4 in the ISO Cube maximum load.
There are three major types of review. The revised list is effective as of November 9 1998. Means a minor or administrative departure from the IRB - approved protocol procedures eg the protocol informed consent document recruitment process or study materials that was made without prior sponsor and IRB.
Revised lists of categories of research activities that may be reviewed by the IRB through the expedited review procedure. Adjustment of status is the way in which an eligible individual who is in the US can become a permanent resident without returning to his or her home country for visa processing US nd. Expedited Review Category 9 Under category 9 an expedited review procedure may be used for the continuing review of research previously approved by the IRB at a convened meeting that meets the following conditions.
Category c9 refers to an EAD applicant who has also filed for adjustment of status ibid 4. Exempt Expedited and Full. FOR FURTHER INFORMATION CONTACT.
Figure 2 Path E1-E2-E3-E4 in the ISO Cube maximum load Robot version Description IRB 1400 for floor mounting IRB 1400CR for clean room installation IRB 1400H for inverted mounting Robot Weight. Categories of Research That May Be Reviewed by the Institutional Review Board IRB through an Expedited Review Procedure. 982 Review of Deviations For studies reviewed under expedited review procedures all major deviations will be reviewed by the convened IRB.
Michele Russell-Einhorn Director of Regulatory Affairs Office for Protection from Research Risks OPRR National. The most common procedures that fit under this category are blood draws that are greater than 2 times per week exposure to low levels of ionizing radiation and skin biopsy. The research does not qualify for Category 9 does not qualify for Category 9.
These new categories are in effective from 21 January 2019. There are 3 categories of review exempt expedited and full board defined by the Federal Regulations for Protection of Human Research Subjects 45 CFR 46. Category 9 means that the IRB can review all subsequent amendments and continuing IRB reviews using expedited procedures.
US 19 May 2010. 2 Expedited Category 9. For protocol deviations that require fully convened IRB review the assigned IRB reviewer will document the determinations and outcomes.
Immigration Attorney 14 May 2010. The determinations and outcomes will be reported in the IRB minutes. In some cases if a study is eligible for expedited review continuing review is not required.
The IRB 1400 is available in three different versions. The research The research Description After using the decision chart above to determine whether a study is eligible to be expedited under Category 9 the following additional items must also be verified. An Institutional Review Board IRB is a review committee established to help protect the rights and welfare of human research subjects.
90 Protocol Violations Deviations and Exceptions 91 Definitions. New Exemption Categories Research involving human subjects is considered exempt if it falls under one of the categories listed below. Exempt research still requires IRB review and approval but is exempt from other requirements such as annual renewal and in some cases informed consent.
When the convened board determines that an application is eligible for review using Category 9 the determination must be documented in the meeting minutes and in ERICA. Continuing review of research not conducted under an investigational new drug application or investigational device exemption where categories 2 through 8 do not apply but the IRB has determined and documented at a convened meeting that the research involves no greater than minimal risk and no additional risks have been identified. The IRB staff.
IRB must review all projects that meet the definition of research and that involve human subjects prior to any data collection to determine the appropriate level of review and as appropriate approve them. CATEGORY 9 Continuing review of research where categories 2 8 do not apply but the IRB has determined and documented at a convened meeting that the research involves no greater than minimal risk and no additional risks have been identified. An explanatory note has been added to categories five and seven to clarify that some research described in these categories may be exempt from the IRB review under 45 CFR 46101 of the HHS.

Marshfield Clinic Research Institute Investigator Resources Human Subject Determinations And Irb Review

The Institutional Review Board What Is An Irb An Irb Is Committee Set Up By An Institution To Review Approve And Regulate Research Conducted Under Ppt Download

Device Review Decision Tree Version Date 4 28 15 This Decision Tree To Be Used For Studies Involving Research On A Device Approved Or Unapproved Ppt Download
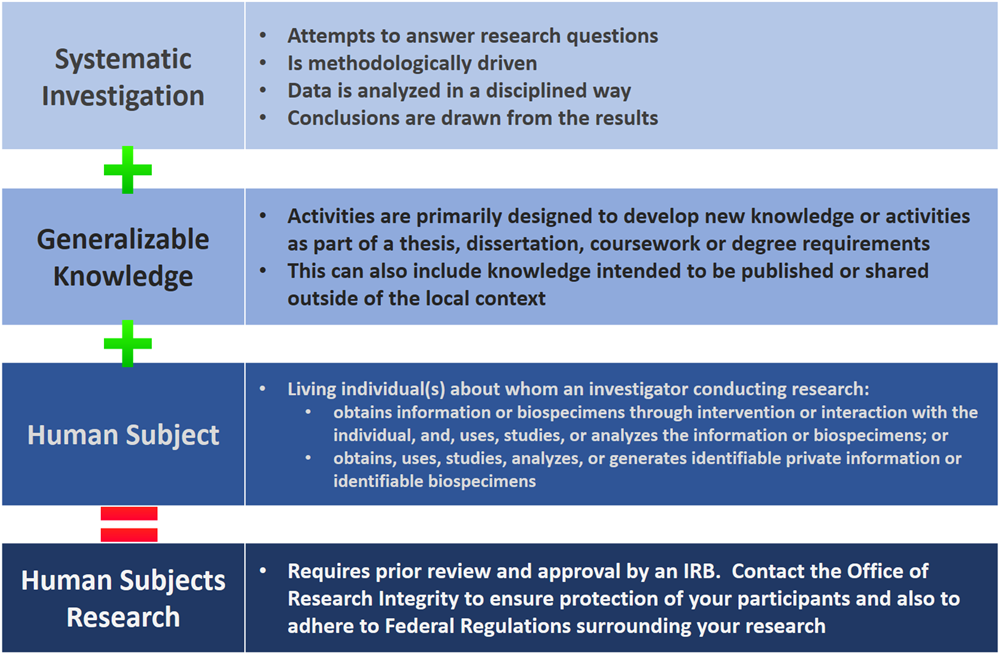
Frequently Asked Questions Institutional Review Board Irb Research And Economic Development
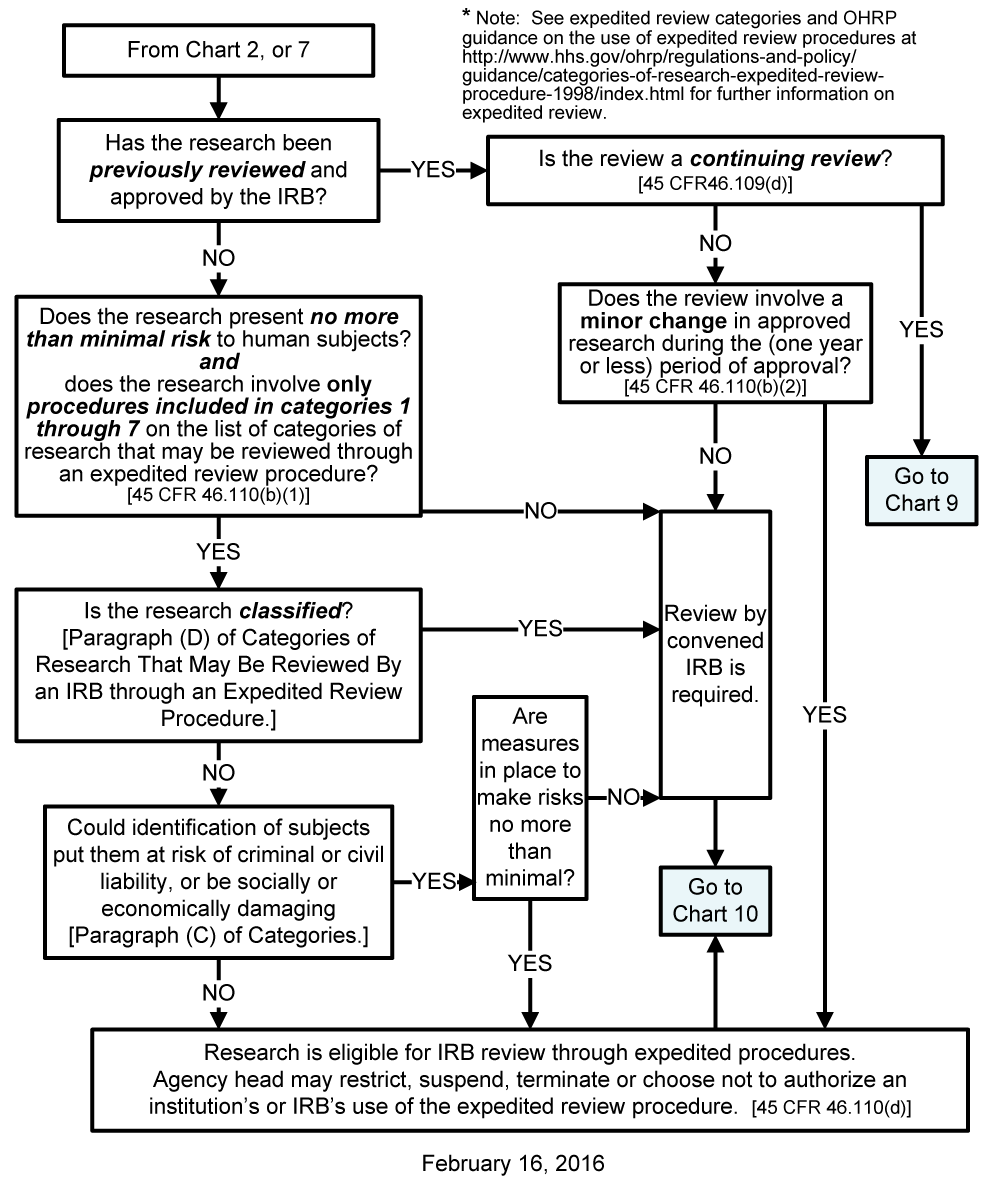
Human Subject Regulations Decision Charts Pre 2018 Requirements Hhs Gov

Ifrs 9 Credit Impairment Wikibanks
Levels Of Review Ucsf Institutional Review Board
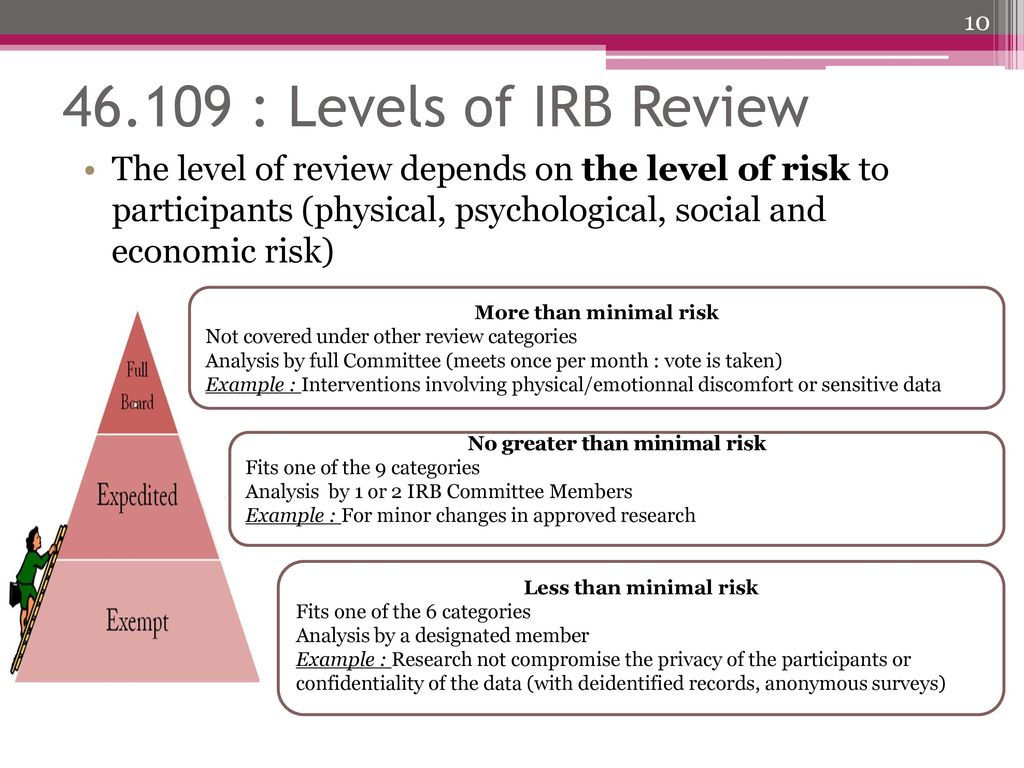
Institutional Review Board Irb Ppt Download

Irb Policies And Procedures Research Protections Office The University Of Vermont
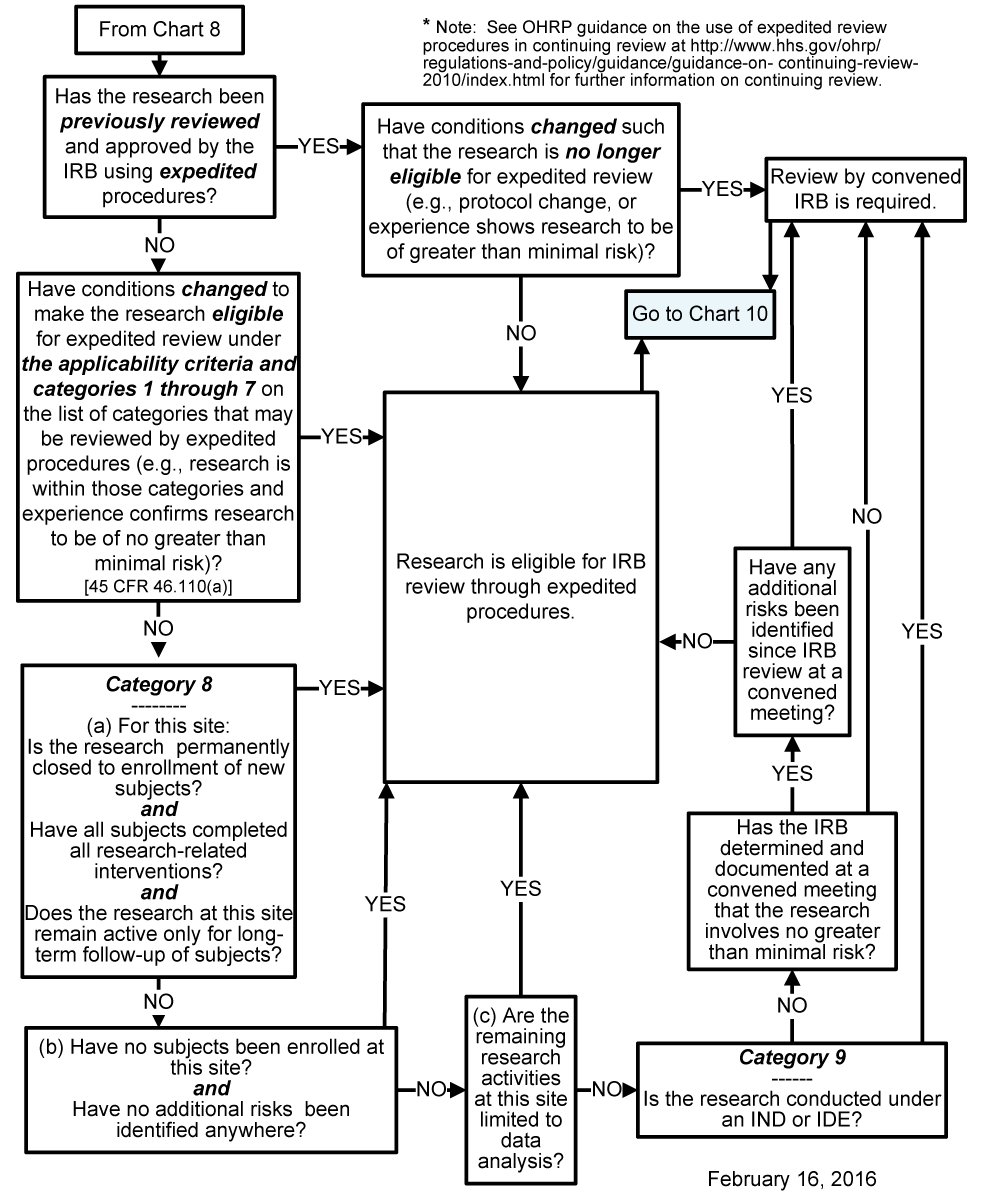
Human Subject Regulations Decision Charts Pre 2018 Requirements Hhs Gov
Conducting Risk Benefit Assessments And Determining Level Of Irb Review Ucla Office Of The Human Research Protection Program
Posting Komentar untuk "Category 9 Irb"