Category A Biological Agents Shipping
Category A pathogens and substances likely to contain Category A pathogens must be assigned the UN number UN2814 proper shipping name. Category A infectious substances.
Biological agent which is defined as the classification of that agent approved by HSE.
Category a biological agents shipping. Exempt Patient Specimens clinical human or animal samples b. Update to identify that the mark in the diamond applied to packages containing biological substances Category B UN 3373 and. Infectious Substance Category A 2.
Infectious substance Category B. In 2006 the USPS stopped accepting Category A shipments although you can ship them via certain specialized carriers. Department of Transportation USDOT andor international agencies IATA ICAO.
Instruction 602 diagrammed below. Exempt Patient specimens clinical human or animal samples 5. The type of shipping paper used in a highway transportation is called a.
Any planned shipment of Select Agents and Toxins must be coordinated with the K-State Responsible. The maximum allowable quantity on passenger aircraft is 50 ml or 50 g. Genetically modified organisms and microorganisms Non-regulated biological materials 4.
These shipments are highly regulated by the US. Biological agents are bacteria viruses parasites and fungi which can cause harm to human health usually due to infection some are toxic or. Below are examples of labels required on biological material packages.
A signed agreement expressly permitting the shipment of Infectious Substance Category A is required. Section 4 Shipping Biologicals UW Shipping Transporting Hazardous Materials Training Shipping a Biological Substance Category B using IATA packing instruction 650. As Biological Substance Category B UN 3373.
Genetically Modified Organisms or Microorganisms 2. Assigned to Category A. Human biological material is defined in section 4 of the Health Research Act as organs parts of organs cells and tissues and components of such material from living and dead persons.
The agent is pathogenic to humans. Human or non-human primate derived materials cell lines blood tissues patient samples etc equipment or instruments that contain hazardous materials. Anthrax Bacillus anthracis Botulism Clostridium botulinum toxin Plague Yersinia pestis Smallpox variola major Tularemia Francisella tularensis Viral hemorrhagic fevers filoviruses eg Ebola Marburg and arenaviruses eg.
The key changes in the 20212022 edition of the Infectious Substances Shipping Guidelines ISSG include. Bacillus anthracis cultures only Brucella abortus cultures only Brucella melitensis cultures only Brucella suis cultures only. A truck papers B freight bill.
Biological Substances Category B 3. In the table in Annex 2 the microorganisms written in italics are bacteria mycoplasmas rickettsiae or fungi. Unregulated biological material.
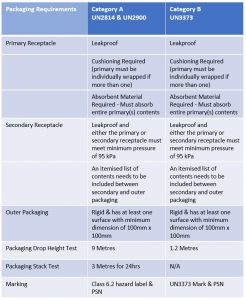
In addition if there is doubt as to whether or not a substance meets the criteria it shall be included in Category A. Infectious Substance Affecting Animals. Use primary receptacles made of glass metal or plastic with a positive means of ensuring a leakproof.
Which category of biological agents is considered high priority and includes toxins that pose the highest risk to the public and national security. For shipment purposes biological material will fit into one of the following categories. Infectious Substances Category A 2.
The maximum quantity of Category A infectious substance that can be shipped by air in one package is 4 L or 4 kg. Primary watertight inner receptacle. The agent is a hazard to employees.
Infectious substances in Category B must be assigned to UN 3373 and their proper shipping name is Biological Substances Category B. Other non-regulated biological material 3. A Category A B Category B.
Individuals shipping Category A infectious substances should contact K-State Environmental Health and Safety. The proper shipping name for Category A is. The Approved List is the list of classifications of biological agents approved by HSE for this purpose.
Addition of a new entry and conditions for solid medical waste containing Category A pathogens. A declaration is not required for shipments in which dry ice is the. Shipping Papers A Shippers Declaration for Dangerous Goods must be completed when shipping a Category A infectious substance or a Genetically Modified Organism or Microorganism assigned to UN 3245.
Infectious Substance Affecting Humans or UN2900 proper shipping name. Enough extra space to hold absorbent and cushioning materials around the primary receptacle. Biological Substance Category B 3.
Senders name and address. General Packaging Requirements For Biological Substance Category B UN 3373 shipments cushioning material is required for both liquid and dried specimens. The outer container of al l Category A infectious substance packages must display the following on two opposite sides.
Category B A Category B substance is an infectious substance that does not meet the criteria for inclusion in Category A. Chemicals in small quantities a. Infectious Substance affecting humans Indicative examples of Category A Infectious Substances are listed below.
Department of Agriculture under 9 CFR Part 21. Category A agents are listed below. If a Category A pathogensubstance is capable of causing disease in both humans and animals the.
You must include four layers of packaging. Classification of Biological Agents. Label information must include the category of the infectious biological material or agent ie.
If your material is infectious but doesnt meet the criteria for inclusion in Category A then you can. The classification system is based on whether. Category A Category B or exempt human or animal specimen.
Service only available within the US. Biological Agents are classified in the Code of Practice to the Safety Health and Welfare at Work Biological Agents Regulations 2013 and 2020 into four risk groups groups 1 2 3 and 4. Classification What are you shipping 1.
Service is not available for Select Agents or Toxins as regulated by the Centers for Disease Control and Prevention under 42 CFR Part 73 or the US. What is human biological material. The course does not provide the details and competency-based assurance to meet DOTIATA training requirements for Category A shipments.
Classification What are you shipping Regulated biological materials.

Commercial Invoice Templates 16 Free Printable Xlsx Word Samples Formats Examples Invoice Template Invoice Template Word Templates

Pin By Danaus Lpp On Beetles Beetle Lady Beetle Guelph

Infectious Substances Packaging Explained Air Sea Containers

Pin On Principles Of Chemotherapy
Infectious Substances Packaging Explained Air Sea Containers

Commercial Invoice Templates 16 Free Printable Xlsx Word Samples Formats Examples Invoice Template Invoice Template Word Templates

Un3373 Compliance A Simple Guide Intelsius Cold Chain Solutions
Un3373 Compliance A Simple Guide Intelsius Cold Chain Solutions

Un3373 Category B Shipping Kit


Posting Komentar untuk "Category A Biological Agents Shipping"